Abstract
Introduction: Controlling both GVHD and disease relapse is essential for allogeneic hematopoietic cell transplantation (alloHCT) success. For three decades, the standard GVHD prophylaxis strategy has been a calcineurin inhibitor such as tacrolimus (Tac) plus methotrexate (MTX). Although several approaches have been tried in recent years to improve upon Tac/MTX, intensifying immunosuppression frequently comes with an increased risk of serious infections or relapse and usually no effect on chronic GVHD even if acute GVHD is reduced. The Blood and Marrow Transplant Clinical Trials Network (BMT CTN) previously completed a phase II study comparing three novel GVHD prophylaxis regimens to contemporaneous controls receiving Tac/MTX (BMT CTN 1203) in order to determine the most promising novel GVHD prophylaxis approach with reduced intensity conditioning (RIC). The most promising GVHD prophylaxis regimen in BMT CTN 1203 was a 3-drug combination of post-transplant cyclophosphamide (PTCy), tacrolimus, and mycophenolate mofetil (PTCy/Tac/MMF). Here, we report the results of the randomized phase III study comparing outcomes of alloHCT in those randomized to receive PTCy/Tac/MMF versus standard Tac/MTX.
Patients and Methods: Eligible adults (age 18+) with hematologic malignancies undergoing RIC alloHCT with an 6/6 matched related (N=128), 8/8 matched unrelated (N=288), or 7/8 single mismatch (N=15) peripheral blood stem cell donor, satisfactory organ function, and adequate performance status were randomized 1:1 to receive PTCy/Tac/MMF (N=214) or Tac/MTX (N=217), stratified by transplant center and Disease Risk Index (DRI). The primary endpoint of the study was GVHD/relapse or progression-free survival (GRFS), a time-to-event outcome defined as grade III-IV acute GVHD, chronic GVHD requiring systemic immune suppression, disease relapse or progression, or death by any cause. The primary hypothesis was that PTCy/Tac/MMF has a ≥15% higher GRFS at 1 year than Tac/MTX in the intent-to-treat population. Secondary endpoints included the incidence/severity of acute and chronic GVHD, engraftment/chimerism, relapse/progression, infections, and survival.
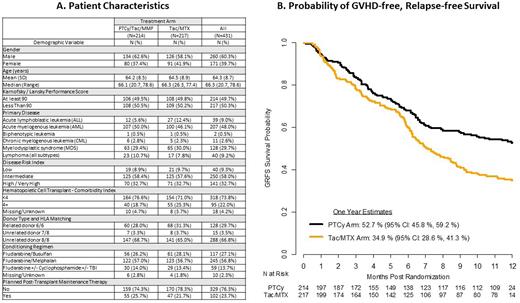
Results: The 2 study arms were well balanced by patient sex, age, Karnofsky performance status, disease risk, comorbidities, donor match, conditioning regimen, and post-transplant maintenance therapy (Figure panel A). In the multivariate Cox regression model of the primary endpoint, the PTCy treatment group had a significantly lower hazard of GRFS than Tac/MTX (hazard ratio 0.641, 95% confidence interval [CI] 0.492 to 0.835, p=0.001). The adjusted 1-year GRFS rate was 52.7% (95% CI: 45.8%, 59.2%) for the PTCy arm and 34.9% (95% CI: 28.6%, 41.3%) for the control arm, Figure panel B). The lower proportion of GRFS events in the PTCy arm was due to a reduction in both acute and chronic GVHD. The Day 100 grade III-IV acute GVHD was 6.3% vs 14.7% (p=0.001), and chronic GVHD rate at 1 year was 21.9% vs 35.1% (p=0.005) for PTCy vs Tac/MTX, respectively. There was no difference in the relapse/progression rate at 1 year (20.8% vs 20.2%, p=0.9), or OS rate at 1 year post transplant (76.8% vs 72.6% , p=0.3), in PTCy vs Tac/MTX. The cumulative incidence of engraftment was lower for PTCy for neutrophils ≥ 500/mm3 by day +28 (90.3% vs 93.4%, p=0.03), platelets ≥ 50,000/mm3 by day +100 (79.5% vs 83.7%, p<0.001), and lymphocytes ≥ 1000/mm3 by 1 year (47.1% vs 63.2%, p<0.001). Grade 3 infection rates were similar between the arms (12.2% for PTCy vs 13.3%, p=0.8) but grade 2 infections were greater for PTCy (33.7% vs 23.5%, p=0.002). There was no difference in CMV reactivation between the treatment arms (7.3% for PTCy vs 7.1%, p=0.8). There was no significant difference in proportion of chimerism at day +100 (>95% donor in 68.6% for PTCy vs 67.8%, p=0.2) or secondary graft failure between the arms (2.9% for PTCy vs 0.9%, p=0.2).
Conclusions: BMT CTN 1703 met its primary endpoint, demonstrating a higher 1-year GRFS with PTCy/Tac/MMF compared to Tac/MTX owing to significant improvements in GVHD risk without increased risk of relapse or death. PTCy/Tac/MMF, which has become standard of care for mismatched transplants, should also become the standard of care for GVHD prophylaxis from closely-matched donors receiving reduced intensity conditioning.
Disclosures
Holtan:Incyte: Research Funding; Vitrac Therapeutics: Research Funding; CSL Behring: Other: Clinical trial adjudication. Hamadani:Genmab: Consultancy; Kite: Consultancy; Medical University of Wisconsin: Current Employment; Incyte Corporation: Consultancy; MorphoSys: Consultancy; SeaGen: Consultancy; Gamida Cell: Consultancy; Novartis: Consultancy; Legend Biotech: Consultancy; Kadmon: Consultancy; ADC Therapeutics: Consultancy, Research Funding, Speakers Bureau; Omeros: Consultancy; Abbvie: Consultancy; Takeda: Research Funding; Spectrum Pharmaceuticals: Research Funding; Astellas Pharma: Research Funding; Sanofi Genzyme: Speakers Bureau; AstraZeneca: Speakers Bureau; BioGene: Speakers Bureau. Runaas:Servier: Honoraria. Elmariah:Bristol Myers Squibb: Research Funding. Larkin:Gilead: Membership on an entity's Board of Directors or advisory committees; ASCO: Consultancy. Shaffer:Miltenyi Biotec: Research Funding; Gamida Cell: Consultancy; Hansa Biopharma: Consultancy. Solh:Partner Therapeutics: Research Funding; ADC Therapeutics: Research Funding. Alousi:Genetech: Consultancy; Sanofi / Kadmon: Honoraria; Mallinkrodt: Honoraria; Prolacta: Consultancy; Incyte: Honoraria, Research Funding. Perales:Celgene: Honoraria; Astellas: Honoraria; Sellas Life Sciences: Consultancy; AbbVie: Honoraria; Novartis: Honoraria; Nektar Therapeutics: Consultancy, Honoraria; Merck: Consultancy; Omeros: Consultancy; Bellicum: Honoraria; Karyopharm: Honoraria; Miltenyi Biotec: Consultancy, Honoraria; Orca Bio: Consultancy; Vor Biopharma: Honoraria; VectivBio AG: Honoraria; DSMB: Other; Cidara Therapeutics: Consultancy; Servier: Consultancy; MorphoSys: Consultancy, Honoraria; Takeda: Honoraria; Medigene: Consultancy; Kite, a Gilead Company: Honoraria, Research Funding; Incyte: Honoraria, Research Funding; Bristol Myers Squibb: Honoraria. Kean:Bristol Myers Squibb: Patents & Royalties: clinical trial, Research Funding; Bluebird Bio: Research Funding; Vertex: Consultancy; Novartis: Research Funding; HiFiBio: Consultancy; EMD-Serono: Research Funding; Mammoth Biosciences: Current equity holder in private company, Current holder of stock options in a privately-held company; Magenta: Research Funding. Efebera:GSK: Honoraria; Takeda: Honoraria; Pfizer: Honoraria; Janssen: Honoraria, Speakers Bureau; Sanofi: Honoraria; BMS: Research Funding. Kitko:Horizon Therapeutics: Consultancy. Reshef:Atara Biotherapeutics: Consultancy, Research Funding; Novartis: Honoraria; Synthekine: Consultancy; Gilead: Consultancy, Honoraria, Other: Travel Support, Research Funding; Incyte: Research Funding; Bayer: Consultancy; MidaTech: Consultancy; TScan: Consultancy; Regeneron: Consultancy; Jasper: Consultancy; Capstan Therapeutics: Consultancy; BMS: Honoraria; University Of Pennsylvania: Other: Data Safety Monitoring Board; Takeda: Research Funding; J&J: Research Funding; Precision Biosciences: Research Funding; Pharmacyclics: Research Funding; Shire: Research Funding; Immatics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal